Tailored Metal-Oxide Supports
Metal oxide supports are ubiquitous in heterogeneous catalysis, acting as a crucial component of catalysts; they enable to disperse metal sites from atomically isolated form, e.g. in Single site or Single Atom Catalysts (SACs), to small metallic clusters or nanoparticles, and also generate interfacial sites that drive catalysis. In that context, numerous support effects have been discussed in catalysis, and our group has focused on providing a molecular-level understanding of support effects across selected reactions such as the hydrogenation of CO2 and the dehydrogenation of propaneas well as dry reforming.[1-5] Utilizing metal-support interactions and tailoring them towards specific reactivity are certainly key to designing more efficient catalytic processes.
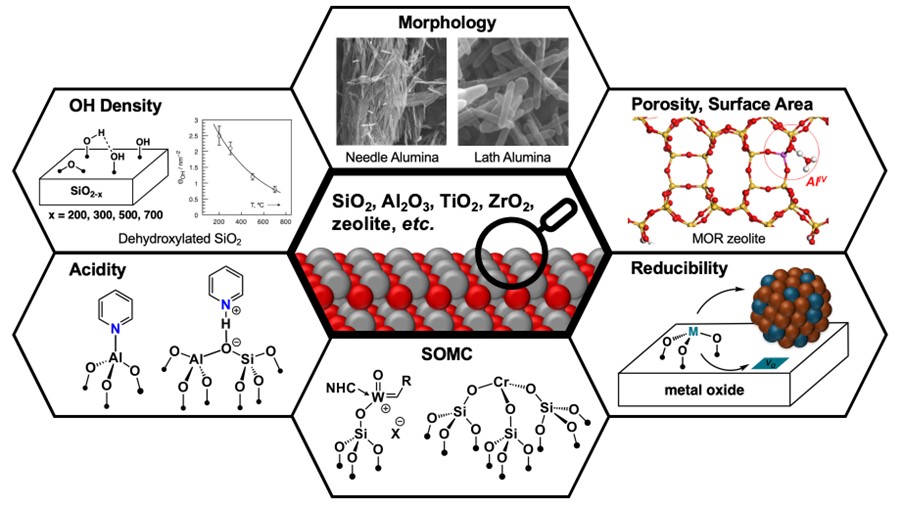
Towards this goal, developing a thorough understanding of tailored metal-oxide supports and the ability to prepare them are both essential. Alumina (Al₂O₃), for instance, is known to exist with different bulk structures depending on the preparation methods, which consequently affect the morphology, the porosity, the surface area, the acidity and basicity and thereby the surface properties of the support material and catalytic performances (Scheme 1).
Our group has thus been interested to prepare tailored supports with controlled surface properties across a range of metal oxide supports, e.g. silica, alumina, zinc oxide, ceria, titania, zirconia, and zeolites. One of the key steps is the preparation of the specific OH density and the type of surface OH groups, in order to control the grafting process and thereby the formation of well-defined active sites/phases (Scheme 1 “OH Density”).
Moreover, the morphology as well as the porosity/surface area can be individually tuned. For example, the morphology of alumina (needles, laths, rhomboids) can be controlled in the solvothermal synthesis by using different capping agents. By controlling the morphology, the aspect ratio of surface facets can be tailored, which exposes preferential grafting sites (Scheme 1 “Morphology”) [7]. Similarly, the surface area of zeolite can be controlled by adjusting the synthesis temperature and concentration (Scheme 1 “Porosity, Surface Area). To evaluate the supports, the properties such as acidity can be probed using specific probe molecules (Scheme 1 “Acidity”), which helps in assessing the effectivness of various anchoring sites. Additinally, these supports enable the reducibility and alloying of active metals in a later stage.
In order to gain deeper understanding of the local coordination environment of the active sites, we also use tailored molecular precursors to generate well-defined surface site mimics. For instance, for silica-based materials, this approach uses molecular precusors e.g. containing a tris(tert-butoxy)siloxide ligands (TBOS) to synthesis [{Cr(OSi(OtBu)3)3}2][8,9,10], [{Zn(TBOS)3}2][11] , [Cu(κ2,μ2-OSi(OtBu)3)(OSi(OtBu)3)]2[12] and Al(TBOS)3[13] mono- and dimers. The same approach could be applied to alumina-based materials, using aluminate precursors such as [Cu(κ2-Al(OtBu)4)2][14]. With these well-defined molecular systems, we can benchmark our spectroscopic assessment and relate the structural properties to the active metal sites on the metal-oxide support. This marks the starting point from where we are able to immobilize active metals on the support (Scheme 1 “SOMC”).
All these preparation and treatment methods allow us to control and fine-tune the physical and chemical properties of the metal-oxide supports, laying the groundwork for molecular-level understanding of their roles in catalytic performances.
Selected Literature
(1) Copéret, C.; Comas-Vives, A.; Conley, M. P.; Estes, D. P.; Fedorov, A.; Mougel, V.; Nagae, H.; Núñez-Zarur, F.; Zhizhko, P. A. Surface Organometallic and Coordination Chemistry toward Single-Site Heterogeneous Catalysts: Strategies, Methods, Structures, and Activities. Chemical Reviews 2016, 116 (2), 323-421. DOI: 10.1021/acs.chemrev.5b00373.
(2) Docherty, S. R.; Copéret, C. Deciphering Metal-Oxide and Metal-Metal Interplay via Surface Organometallic Chemistry: A Case Study with CO2 Hydrogenation to Methanol. J Am Chem Soc 2021, 143 (18), 6767-6780. DOI: 10.1021/jacs.1c02555.
(3) Docherty, S. R.; Rochlitz, L.; Payard, P. A.; Copéret, C. Heterogeneous alkane dehydrogenation catalysts investigated a surface organometallic chemistry approach. Chem Soc Rev 2021, 50 (9), 5806-5822. DOI: 10.1039/d0cs01424a.
(4) Lam, E.; Corral-Prez, J. J.; Larmier, K.; Noh, G.; Wolf, P.; Comas-Vives, A.; Urakawa, A.; Copret, C. CO2 Hydrogenation on Cu/Al2O3: Role of Metal/Support Interface in Driving Activity and Selectivity of a Bifunctional Catalyst. Angew Chem Int Edit 2019, 58 (39), 13989-13996. DOI: 10.1002/anie.201908060.
(5) Rochlitz, L.; Pessemesse, Q.; Fischer, J. W. A.; Klose, D.; Clark, A. H.; Plodinec, M.; Jeschke, G.; Payard, P. A.; Coperet, C. A Robust and Efficient Propane Dehydrogenation Catalyst from Unexpectedly Segregated Pt2Mn Nanoparticles. J Am Chem Soc 2022. DOI: 10.1021/jacs.2c05618.
(6) Yakimov, A. V.; Ravi, M.; Verel, R.; Sushkevich, V. L.; van Bokhoven, J. A.; Copéret, C. Structure and Framework Association of Lewis Acid Sites in MOR Zeolite. J Am Chem Soc 2022, 144 (23), 10377-10385. DOI: 10.1021/jacs.2c02212.
(7) Völker, L. A.; Meyet, J.; Berkson, Z. J.; Rochlitz, L.; van Bokhoven, J. A.; Copéret, C. Revisiting Edge Sites of γ-Al2O3 Using Needle-Shaped Nanocrystals and Recoupling-Time-Encoded {27Al}-1H D-HMQC NMR Spectroscopy. J Phys Chem C 2022, 126 (14), 6351-6360. DOI: 10.1021/acs.jpcc.2c00979.
(8) Conley, M. P.; Delley, M. F.; Siddiqi, G.; Lapadula, G.; Norsic, S.; Monteil, V.; Safonova, O. V.; Copéret, C. Polymerization of Ethylene by Silica-Supported Dinuclear CrIII Sites through an Initiation Step Involving C-H Bond Activation. Angew Chem Int Edit 2014, 53 (7), 1872-1876. DOI: 10.1002/anie.201308983.
(9) Conley, M. P.; Delley, M. F.; Núñez-Zarur, F.; Comas-Vives, A.; Copéret, C. Heterolytic Activation of C-H Bonds on CrIII-O Surface Sites Is a Key Step in Catalytic Polymerization of Ethylene and Dehydrogenation of Propane. Inorg Chem 2015, 54 (11), 5065-5078. DOI: 10.1021/ic502696n.
(10) Delley, M. F.; Núñez-Zarur, F.; Conley, M. P.; Comas-Vives, A.; Siddiqi, G.; Norsic, S.; Monteil, V.; Safonova, O. V.; Copéret, C. Proton transfers are key elementary steps in ethylene polymerization on isolated chromium(III) silicates (vol 111, pg 11624, 2014). P Natl Acad Sci USA 2015, 112 (32), E4505-E4505. DOI: 10.1073/pnas.1512495112.
(11) Rendón, N.; Bourdolle, A.; Baldeck, P. L.; Le Bozec, H.; Andraud, C.; Brasselet, S.; Copéret, C.; Maury, O. Bright Luminescent Silica Nanoparticles for Two-Photon Microscopy Imaging via Controlled Formation of 4,4′-Diethylaminostyryl-2,2′-bipyridine Zn(II) Surface Complexes. Chem Mater 2011, 23 (13), 3228-3236. DOI: 10.1021/cm2010852.
(12) Meyet, J.; Searles, K.; Newton, M. A.; Wörle, M.; van Bavel, A. P.; Horton, A. D.; van Bokhoven, J. A.; Copéret, C. Monomeric Copper(II) Sites Supported on Alumina Selectively Convert Methane to Methanol. Angew Chem Int Edit 2019, 58 (29), 9841-9845. DOI: 10.1002/anie.201903802.
(13) Valla, M.; Stadler, D.; Mougel, V.; Copéret, C. Switching on the Metathesis Activity of Re Oxo Alkylidene Surface Sites through a Tailor-Made Silica-Alumina Support. Angew Chem Int Edit 2016, 55 (3), 1124-1127. DOI: 10.1002/anie.201509390.
(14) Meyet, J.; Ashuiev, A.; Noh, G.; Newton, M. A.; Klose, D.; Searles, K.; van Bavel, A. P.; Horton, A. D.; Jeschke, G.; van Bokhoven, J. A.; et al. Methane-to-Methanol on Mononuclear Copper(II) Sites Supported on Al2O3: Structure of Active Sites from Electron Paramagnetic Resonance**. Angew Chem Int Edit 2021, 60 (29), 16200-16207. DOI: 10.1002/anie.202105307.