Supported Nanoparticles & Bimetallic Catalysts
![Figure 1. Catalyst structural features with potential catalytic influence.[2]](/research/making/nano/_jcr_content/par/image/image.imageformat.1286.1034454022.jpg)
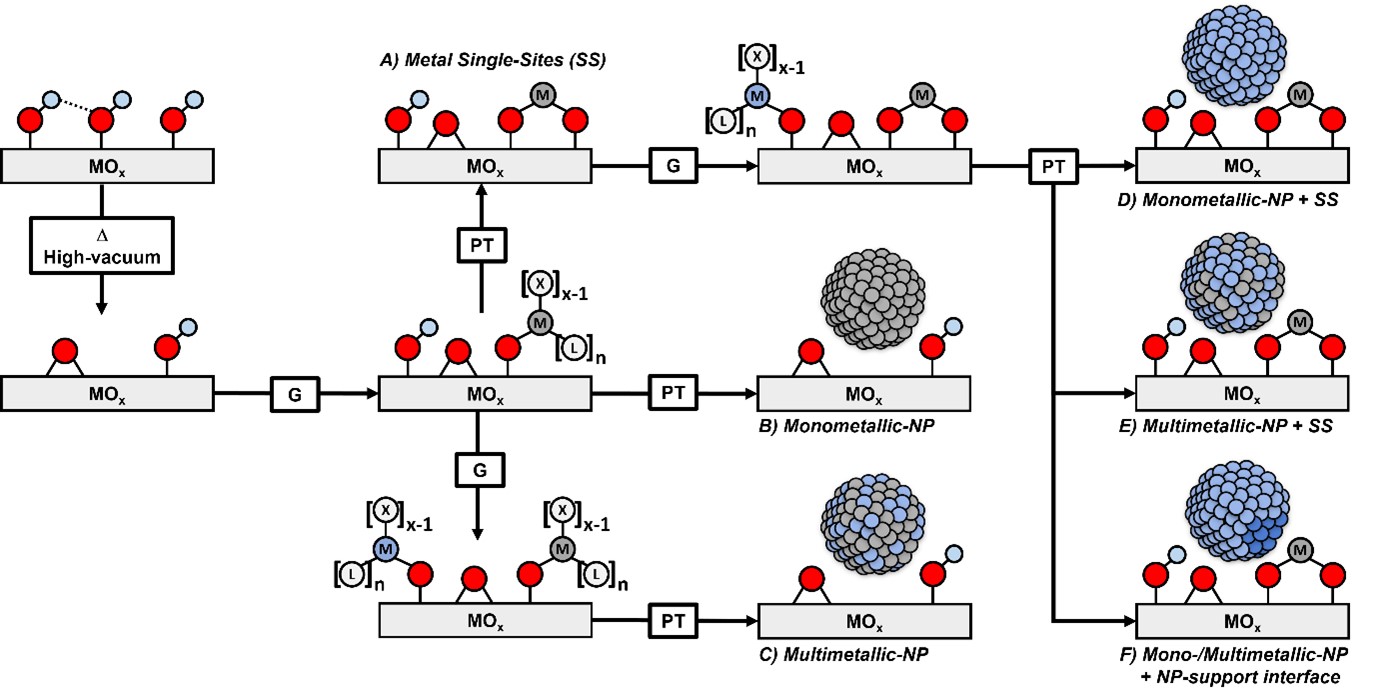
Supported nanoparticles are ubiquitous in catalysis, from academic research to industrial processes. These catalysts are typically prepared via conventional preparation protocols, such as impregnation or precipitation, which often yield ill-defined materials, displaying a broad range of structural motifs due to numerous dissolution and precipitation processes happening in an aqueous environment. In this context, the Copéret group has developed a strategy to syn-thesize tailor-made model catalysts to understand the effect of specific catalyst structural features on catalyst performances, such as catalytic activity, selectivity and stability (see fig. 1). In that context, we use surface organometallic chemistry (SOMC, Figure 2) and tailored molecular precursors and supports, relying on density of surface reactive sites, to control the initial grafting step, composition and surface chemistry with the goal to generate better-defined catalyst structures,[1] such as nanoparticles with controlled interfaces and alloys in the context of multimetallic systems. These catalysts are particularly suited for advanced spectroscopic / microscopic techniques, thus enabling to gain specific understanding on the role of the support or promotional effects in heterogenous catalysis. This methodology has been applied to develop detailed structure-activity relationships across numerous reactions, ranging from the selective hydrogenation of alkynes and COx (methanol and higher alcohol synthesis, WGS or methanation), as well as propane dehydrogenation and hydrodeoxygenation of biomass derivatives.[2-4]
For instance, this methodology was leveraged in a recent study investigating the particle size effect in cobalt-based CO2 hydrogenation, where a series of size-controlled cobalt nanoparti-cles supported on SiO2, ranging from 1.6 – 3.0 nm, were synthesized using the SOMC approach. We found that smaller Co-NPs favor RWGS making predominantly CO, while larger Co-NPs behave classically in forming methane from CO2. Using operando X-Ray absorption spectros-copy, we could link cobalt particle size affecting the product selectivity with the state of cobalt under CO2 hydrogenation conditions. The study revealed that smaller cobalt nanoparticles are more likely to be oxidized, which results in their increased CO product selectivity while larger cobalt nanoparticles remain metallic, retaining their high selectivity for methane.[5]
Selected Literature
[1] C. Copéret, Acc. Chem. Res. 2019, 52, 1697-1708.
[2] S. R. Docherty, C. Copéret, J. Am. Chem. Soc. 2021, 143, 6767-6780.
[3] S. R. Docherty, L. Rochlitz, P.-A. Payard, C. Copéret, Chem. Soc.Rev. 2021, 50, 5806-5822.
[4] L. Huang, D. Li, D. Tian, L. Jiang, Z. Li, H. Wang, K. Li, Energy & Fuels 2022, 36, 5102-5151.
[5] X. Zhou, G. A. Price, G. J. Sunley, C. Copéret, Angew. Chem. Int. Ed. 2023, 62, e202314274.