Decoding NMR Chemical Shift
Chemical Shift contains detailed information about the electronic environment of nuclei. Its value depends on the nature and relative energies of molecular frontier orbitals. Below are discussed a series of case studies from our group, where we have used the power of solid-state NMR augmented by natural chemical shift (computational) analysis to understand chemical shift values in order to decipher the nature of frontier molecular and to predict the reactivity of molecules.
external pageAccess the paper on ACS Central Science.call_made
external pageHear a short explanation on the paper (LiveSlides).call_made
Read a short summary:
DownloadEnglish (PDF, 259 KB)vertical_align_bottom – DownloadFrench (PDF, 264 KB)vertical_align_bottom – DownloadGerman (PDF, 215 KB)vertical_align_bottom
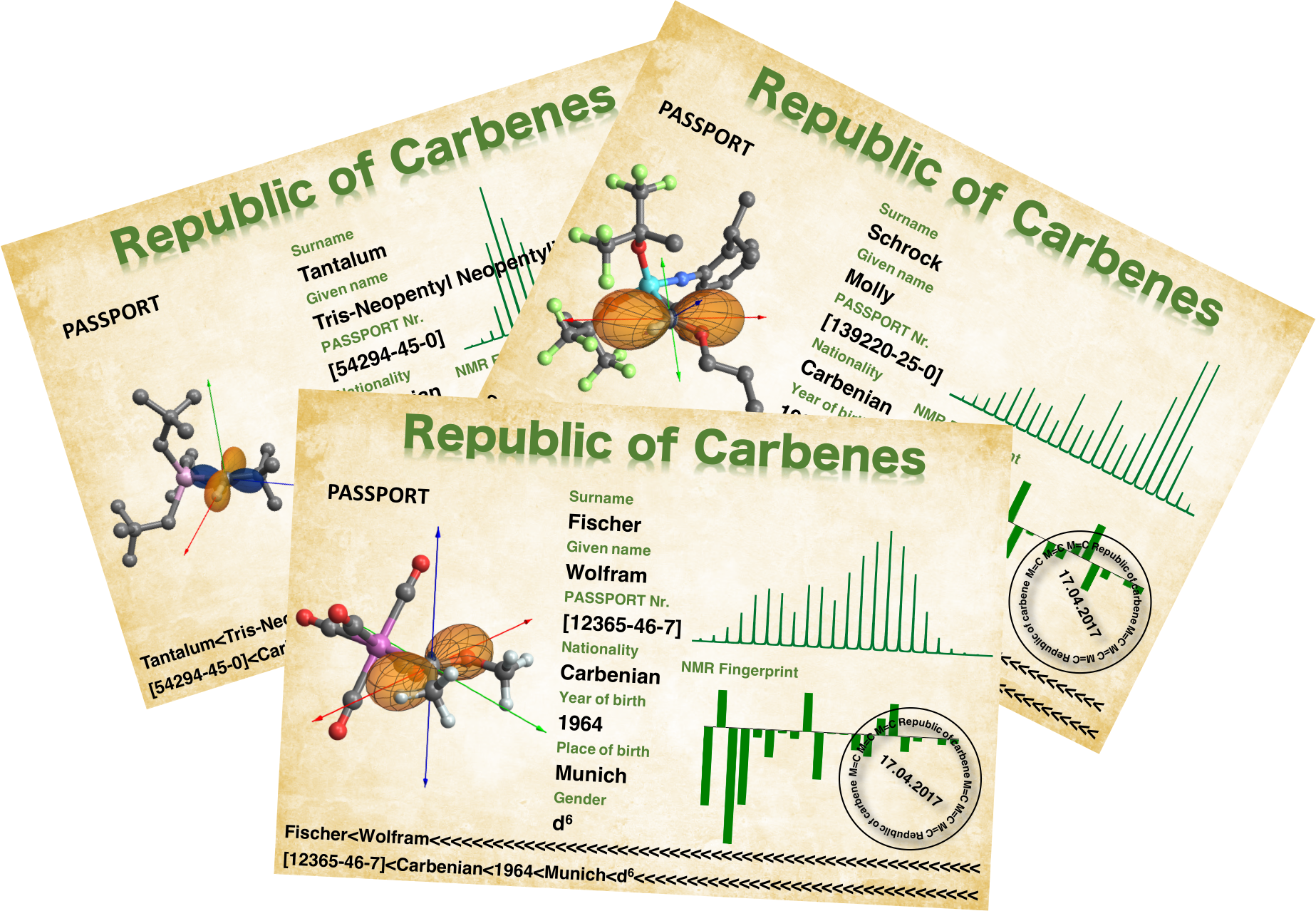
Molecular Fingerprints: Distinguishing Fischer Carbenes and Schrock Alkylidenes
Our research article entitled "Orbital Analysis of Carbon-13 Chemical Shift Tensors Reveals Patterns to Distinguish Fischer and Schrock Carbenes" published in Angew. Chem. Int. Ed. gives insights into the origin of the highly deshielded carbon chemical shift of Fischer and Schrock carbene complexes. In this study, the chemical shift anisotropy of various carbene complexes was determined by solid-state NMR and deconstructed by DFT calculations and natural chemical shift (NCS) analysis. Overall, analysis of the chemical shift tensors revealed fingerprints of the various carbenes, allowing to distinguish Fischer carbenes and Schrock alkylidenes, and relating the chemical shift to reactivity (electrophilic vs. nucleophilic).
Access our papers for more information:
external pageAngew. Chem. Int. Ed. 2017, 56, pp 10127–10131.call_made
external pageJ. Am. Chem. Soc. 2016, 138 (7), pp 2261–2272.call_made
We thus made passports for each carbene complexes.